-
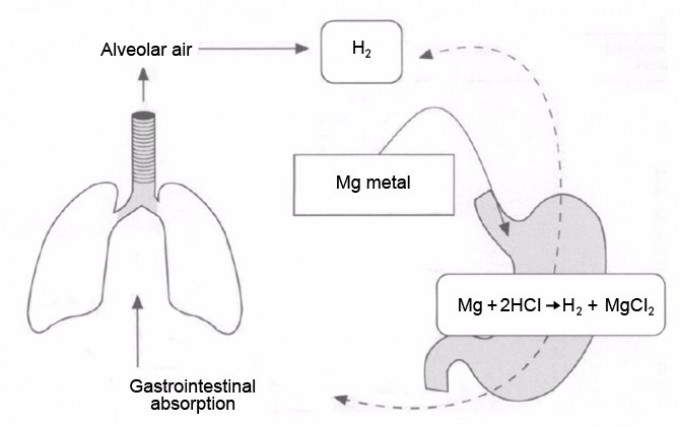
- 01 | 红细胞寿命测定(CO呼气试验)中 高胆红素血症是指血液中胆红素超过正常值的..
- 02 | Breath Analysis Predicts Immun Published on September 16, 2022 Though m..
- 03 | Breath Test Gas Patterns in In Phillip Gu 1 , Devin Patel 2 , Krutika L..
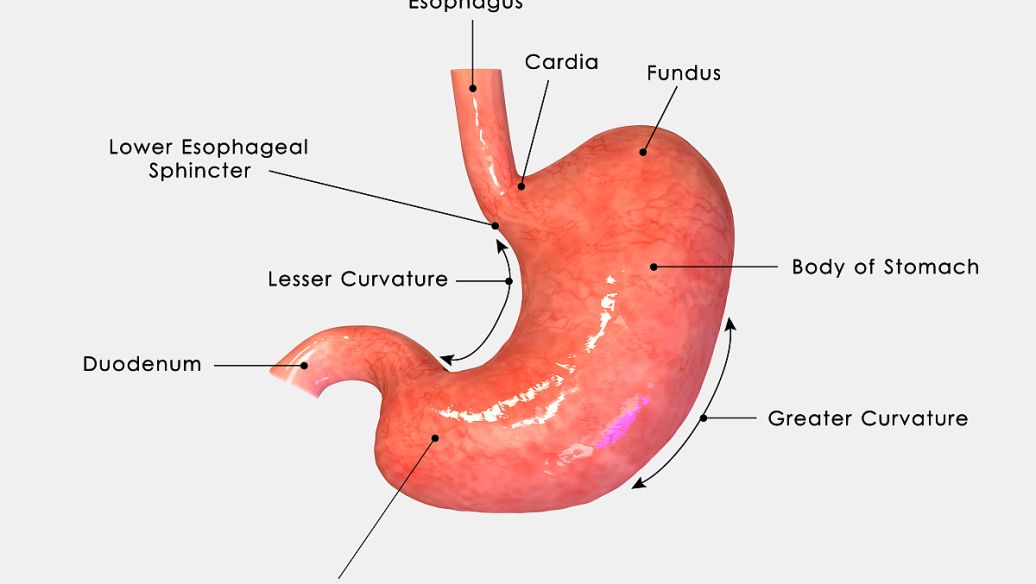
- 04 | SIBO诊断:H2呼气实验 H 2 呼气实验是基于测量呼出气中的氢浓度。..
- 05 | NIH scientists develop breath Researchers at the National Institutes o..
- 06 | 协和深圳医院血液科引进CO呼气试 消息报讯(记者 黄涛 通讯员 李悦)无须采..
-
- 01 | 红细胞寿命测定(CO呼气试验)中 高胆红素血症是指血液中胆红素超过正常值的..
热点推荐
- 01 | 中科院无创筛查获新突破!做个深 食管癌是一种常见的恶性肿瘤,在全球范围内..
- 02 | 先亚集团研发的高新科技产品红细 一、概述 通过呼气测定来进行人体红细胞寿..
- 03 | Capnia Inc公司研发的便携式呼气 美国Capnia Inc公司研发了一款便携式呼气末..
- 04 | 以色列新型仪器用呼气成分诊断疾 我们身体“运行”的过程中会产生许多代谢物..
Breath Analysis Predicts Immunotherapy Success for Mesothelioma Patients
Though much remains to be known about treating and curing malignant mesothelioma, researchers are certain that the most effective treatments are those that are based on the patient’s unique biophysical profile. A recent study conducted at the University of Amsterdam confirmed a breath test’s predictive ability for assessing whether immunotherapy will be effective for individual patients.

eNose Breath Test Analyzes Biomarkers in Mesothelioma Patients’ Breath
Immunotherapy is a relatively new and exciting treatment for malignant pleural mesothelioma. But the treatment’s effectiveness has been spotty, with some patients responding well and others experiencing disappointing results. A study conducted by Dutch researchers has identified biomarkers in patients’ breath that can predict which patients will benefit and which are less likely to do so.
The technology that was tested is called eNose, a breath test that looks for biomarkers in mesothelioma patients’ breath, identifying profiles that differ in those that respond to immunotherapy treatment and those that don’t. The test looks for volatile organic compounds (VOCs) that measure cells’ metabolism and the amount of disease that is present.
Breath Test Was Previously Proven 74% Accurate in Distinguishing Mesothelioma
The VOCs that are present in patients’ breath can do more than predict which patients will respond to immunotherapy and which won’t. Previous studies have confirmed that the same eNose technology has a 74% accuracy score in distinguishing between patients who have malignant pleural mesothelioma and those with other asbestos-related diseases.
Knowing which mesothelioma patients are likely to respond to immunotherapy, and which won’t, frees physicians to provide the best possible care to each patient based on their individual biophysical profile. The report concluded, “An eNose is able to discriminate at baseline between responders and non-responders to nivolumab plus ipilumumab in malignant pleural mesothelioma, thereby potentially identifying a subgroup of patients that will benefit from immune checkpoint inhibitor treatment.”
If you or someone you love has been diagnosed with malignant mesothelioma, innovations in treatment offer the possibility of better quality of life and survival. For more information, contact the Patient Advocates at Mesothelioma.net today at 1-800-692-8608.